安紀堯
安紀堯 Ji-Yao An
frej14862@gmail.com
2020 MS Tunghai University
Current Job: DuPont Taiwan
2021
| 73. | S. B. Patil, J.-Y. An, Z.-J. Li, Y.-C. Wu, S. M. Gowdru, H.-H. Hsieh, Z. Chen, and Di-Yan Wang* 2021: Cost-Effective 1T-MoS2 Grown on Graphite Cathode Materials for High-Temperature Rechargeable Aluminum Ion Batteries and Hydrogen Evolution in Water Splitting, Catalysts 2021, 11(12), 1547(9pp). (invited research article) |
| 72. | Y.-H. Lai*, S.-R. Li, S. M G, H.-T. Chang, Y.-B. Huang, Y.-K. Li, Y.-M. Chen, S. B. Patil, S.-Y. Chang, P.-K. Chen, C.-C. Chang, Y.-C. Chen, C.-W. Pao, J.-L. Chen, C.-Y. Wei, I-K. Lin, H.-L. Chou, C.-J. Su, U-S. Jeng, T.-R. Kuo, C.-Y. Wen, and Di-Yan Wang* 2021: Enhanced Hydrogen Evolution Efficiency Achieved by Atomically Controlled Platinum Deposited on Gold Nanodendrites with High-Index Surfaces, J. Mater. Chem. A, 2021, 9(40), 22901-22912. |
| 71. | B. W. Taklu, W.-N. Su*, Y. Nikodimos, K. Lakshmanan, N. T. Temesgen, P.-X. Lin, S.-K. Jiang, C.-J. Huang, Di-Yan Wang, H.-S. Sheu, S.-H. Wu,* and B.-J. Hwang* 2021: Dual CuCl doped argyrodite superconductor to boost the interfacial compatibility and air stability for all solid-state lithium metal batteries, Nano Energy, 2021, 90, 106542. |
| 70. | J.-M. Meng, Z.-X. Yang, S. B. Patil, J.-C. Lin, C.-H. Yeh, Y.-C. Chen, C.-W. Pao, J.-L. Chen, W.-Y. Chen, C.-W. Lu, T.-R. Kuo, and Di-Yan Wang* 2021: Facile Fabrication of Highly-Stable and Wavelength-Tunable Tin based Perovskite Materials with Enhanced Quantum Yield via Cation Transformation Reaction, J. Phys. Chem. Lett., 2021, 12(36), 8763-8769. |
| 69. | S. B. Patil, T.-R. Liu, H.-L. Chou*, Y.‐B. Huang, C.-C. Chang, Y.-C. Chen, Y.-S. Lin, H. Li, Y.-C. Lee, Y. J. Chang, Y.-H. Lai, C.-Y. Wen, and Di-Yan Wang* 2021: Electrocatalytic Reduction of NO3– to Ultrapure Ammonia on {200} Facet Dominant Cu Nanodendrites with High Conversion Faraday Efficiency, J. Phys. Chem. Lett., 2021, 12(33), 8121-8128. (as Supplementary cover) |
| 68. | C. Mutalik, D. I. Krisnawati, Shivaraj B. Patil, M. Khafid, D. S. Atmojo, P. Santoso, S.-C. Lu, Di-Yan Wang,* and T.-R. Kuo* 2021: Phase-Dependent MoS2 Nanoflowers for Light-Driven Antibacterial Application, ACS Sustain. Chem. Eng., 2021, 9(23), 7904–7912. |
| 67. | Y.-S. Lin, H. Li, W.-S. Yu, S.-T. Wang, Y.-M. Chang, T.-H. Liu, S.-S. Li*, M. Watanabe, H.-H. Chiu, Di-Yan Wang, and Y. J. Chang* 2021: [2.2]Paracyclophane-Based Hole-Transporting Materials for Perovskite Solar Cells, J. Power Sources, 2021, 491, 229543(9pp). |
| 66. | T.-P. Chen, J.-X. Lin, C.-C. Lin, C.-Y. Lin, W.-C. Ke, Di-Yan Wang, H.-S. Hsu*, C.-C. Chen, and C.-W. Chen* 2021: Strong Excitonic Magneto-Optic Effects in Two-Dimensional Organic−Inorganic Hybrid Perovskites, ACS Appl. Mater. Interfaces, 2021, 13(8), 10279−10286. |
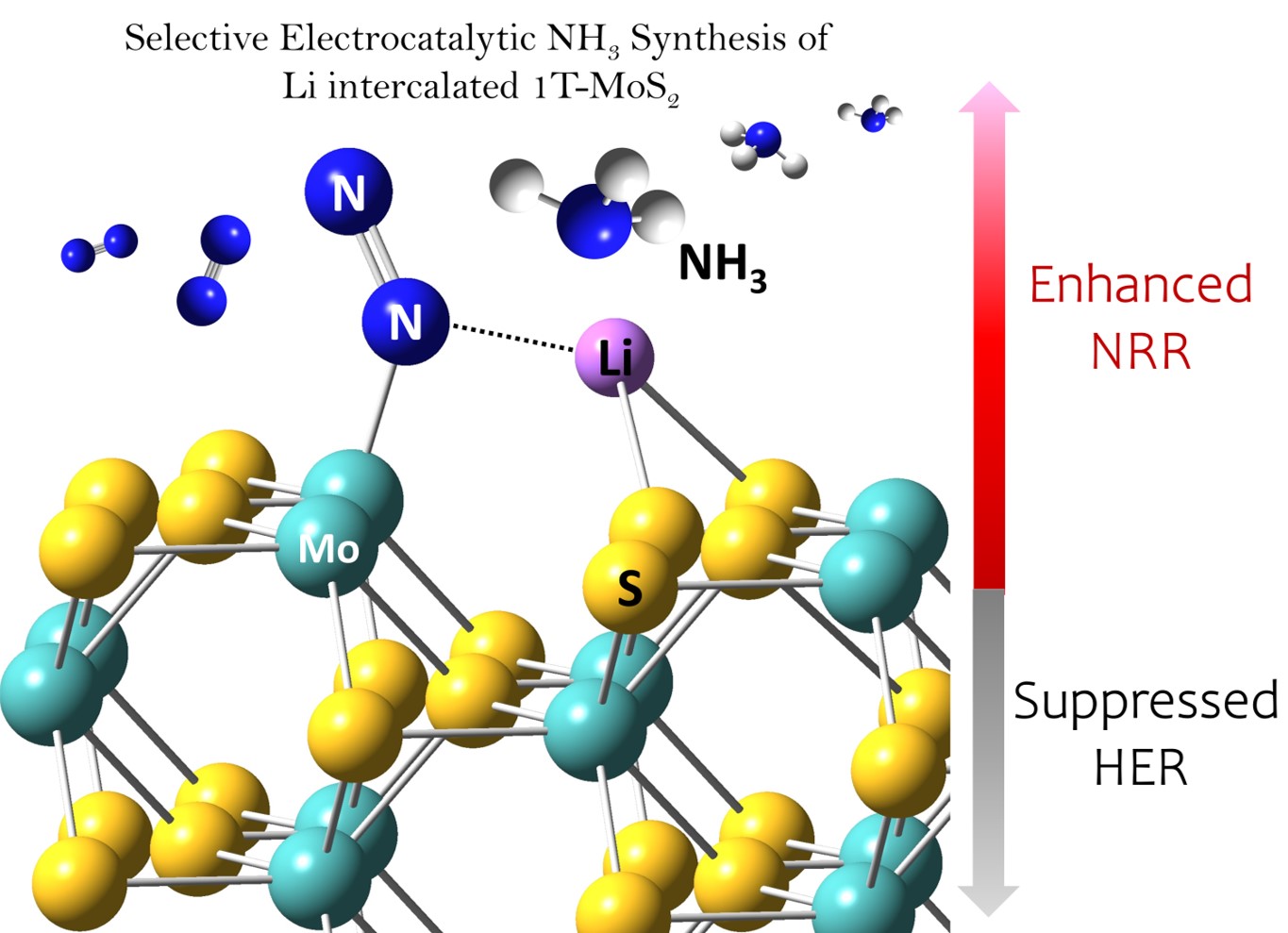
| 65. | S. B. Patil, H.-L. Chou, Y.-M. Chen, S.-H. Hsieh, C.-H. Chen, C.-C. Chang, S.-R. Li, Y.-C. Lee, Y.-S. Lin, H. Li, Y. J. Chang, Y.-H. Lai, and Di-Yan Wang* 2020: Enhanced N2 Affinity of 1T-MoS2 with Unique Pseudo Six-membered Ring Consisting of N—Li—S—Mo—S—Mo for High Ambient Ammonia Electrosynthesis Performance, J. Mater. Chem. A, 2021, 9(2), 1230–1239. |
04 Dec. 2020, our work “Enhanced N2 Affinity of 1T-MoS2 with Unique Pseudo Six-membered Ring Consisting of N—Li—S—Mo—S—Mo for High Ambient Ammonia Electrosynthesis Performance” has been accepted by JMCA
Enhanced N2 Affinity of 1T-MoS2 with Unique Pseudo Six-membered Ring Consisting of N—Li—S—Mo—S—Mo for High Ambient Ammonia Electrosynthesis Performance
Shivaraj B. Patil,1#Hung-Lung Chou,2# Yu-Mei Chen,1 Shang-Hsien Hsieh,3 Chia-Hao Chen,3 Chia-Che Chang,1 Shin-Ren Li,1 Yi-Cheng Lee,1 Ying-Sheng Lin,1 Hsin Li,1 Yuan Jay Chang,1 Ying-Huang Lai,1 Di-Yan Wang1*
https://doi.org/10.1039/D0TA10696H
The Haber–Bosch process is widely used to convert atmospheric nitrogen (N2) into ammonia (NH3). However, the extreme reaction conditions and abundant carbon released by this process make it important to develop a greener NH3 production method. The electrochemical nitrogen reduction reaction (NRR) is an attractive alternative to the Haber–Bosch process. Herein, we demonstrated that molybdenum sulfide on nickel foil (1T-MoS2-Ni) with low crystallinity was an active NRR electrocatalyst. 1T-MoS2-Ni achieved a high faradaic efficiency of 27.66% for the NRR at −0.3 V (vs. RHE) in LiClO4 electrolyte. In-situ X-ray diffraction and ex-situ X-ray photoemission analyses showed that lithium ions intercalated into the 1T-MoS2 layers during the NRR.Moreover, theoretical calculations revealed the differences between six membered rings formed in the 1T-MoS2 and 2H-MoS2 systems with Li intercalation. The bond distances of d(Mo—N) and d(N—Li) of in Li-1T-MoS2 were found to be shorter than those in Li-2H-MoS2, resulting in a lower energy barrier of N2 fixation and higher NRR activity. Therefore, 1T-MoS2-Ni is promising as a scalable and low-cost NRR electrocatalyst with lower power consumption and carbon emission than the Haber–Bosch process.
15 Oct. 2020, our work “Challenges and Prospects of Polyatomic Ions Intercalation in the Graphite Layer for Energy Storage Applications” has been accepted by Phys. Chem. Chem. Phys.
Challenges and Prospects of Polyatomic Ions Intercalation in the Graphite Layer for Energy Storage Applications
Shivaraj B. Patil, Hsiang-Ju Liao and Di-Yan Wang*
https://doi.org/10.1039/D0CP04098C
Population explosion has led to the rapid revolution of science and technology. High energy demand has urged for new and efficient energy conversion and storage systems. Lithium ion batteries (LIBs) have high potential window, high capacity and high stability but it suffers from high cost and low safety. Therefore, many alternative batteries including sodium (NIBs), potassium (KIBs), aluminum (AIBs) and dual ion batteries (DIBs) have been introduced. One of working principle of these batteries is based on cation or anion intercalation in the graphite layers, which are known as formation of graphite intercalation compounds (GICs). Recently, the studies based on reaction mechanism to improve the performance of the batteries have been elucidated. In this review, a view on reaction mechanism of polyatomic ions intercalated into GICs, structure of intercalated polyatomic ions, structure of accommodated GICs and staging are provided. The current limitations and our prospects on polyatomic ions intercalated batteries are also discussed.
